Have you ever wondered about the tiny molecules that lurk in some of the foods you consume and the household items you use? These molecules are called oxalates, and they can be found in many places you might not expect. Understanding the chemistry of oxalates not only reveals the beauty and complexity of the natural world, but also sheds light on how these compounds impact our daily lives—from our dietary choices to the health of our body’s organs.

What are Oxalates?
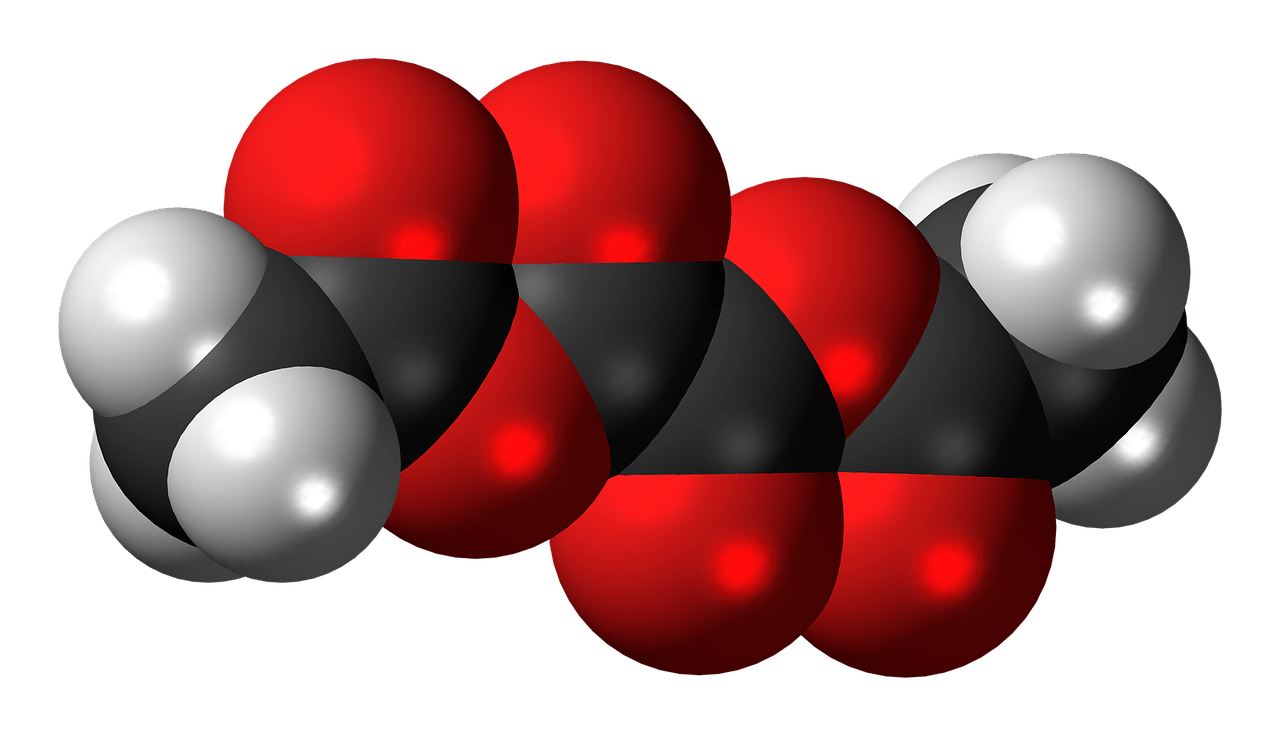
Oxalates are organic compounds that occur naturally in many plants. They are salt or ester derivatives of oxalic acid and have the chemical formula (C_2O_4^{2-}). The presence of oxalates in plants serves various roles, but most notably, they can bind to minerals, which can have implications for both plant and human health.
The Role of Oxalates in Plants
Plants often use oxalates for defense. These compounds can deter herbivores and pests due to their potential toxicity or ability to form crystals. Oxalate crystals can disrupt cell membranes or cause physical damage to the digestive tracts of small insects and mammals. This serves as a protective mechanism to ensure the survival of the plant.
Health Implications for Humans
For humans, oxalates are a double-edged sword. On one hand, they can bind with minerals to form compounds like calcium oxalate, which is insoluble and can precipitate out of solution. When this happens in the kidneys, it may lead to the formation of kidney stones, a painful condition experienced by many. On the other hand, in moderate amounts, oxalates play a role in normal bodily functions and contribute to metabolic processes.
The Chemical Structure of Oxalates
Understanding the chemical structure of oxalates helps in grasping their function and behavior. Oxalates consist of two carbon atoms, four oxygen atoms, and carry a negative charge. This configuration allows them to readily bind with positively charged ions such as calcium, magnesium, and iron.
Formation of Calcium Oxalate
Calcium oxalate is one of the most prevalent forms found in nature and in the human body. Below is a simple chemical equation showing the formation of calcium oxalate:
[ \text{Ca}^{2+} + C_2O_4^{2-} \rightarrow \text{CaC}_2\text{O}_4 ]
This reaction explains why diets high in oxalates combined with calcium can lead to stone formation.

Dietary Sources of Oxalates
Oxalates are widespread in a variety of foods. Some foods are notably higher in oxalates, and consuming them in large quantities could increase your risk of issues like kidney stones, especially if predisposed. Here, we’ll look at common foods containing oxalates and some considerations to keep in mind when planning a balanced diet.
| Food Type | High Oxalate Foods | Moderate Oxalate Foods | Low Oxalate Foods |
|---|---|---|---|
| Vegetables | Spinach, rhubarb | Sweet potatoes, Swiss chard | Broccoli, kale |
| Fruits | Starfruit, figs | Oranges, raspberries | Bananas, apples |
| Nuts & Seeds | Almonds, peanuts | Pine nuts, cashew nuts | Sunflower seeds, pecans |
| Beverages | Tea | Coffee | Herbal tea (non-oxalate) |
Impact of Cooking on Oxalates
Cooking can alter the oxalate content of foods. Boiling, for instance, can leach soluble oxalates into the cooking water, reducing the overall oxalate content. Steaming or roasting vegetables, however, might not be as effective. Understanding how food preparation impacts oxalate levels can help you make informed dietary choices.
Considerations for Special Diets
If you have dietary concerns like a predisposition to kidney stones, a low-oxalate diet might be considered under medical advice. balancing your diet with an awareness of oxalate content can help in managing your health effectively. It’s important to keep a varied diet and not simply avoid high oxalate foods entirely, since many contain other vital nutrients.
Oxalates and the Human Body
Once in your system, oxalates don’t simply disappear; they have significant interactions and can influence your health.
Absorption and Elimination
Your body absorbs oxalates through the gut, but only a fraction (typically 10-15%) of dietary oxalate is absorbed. The rest passes out through the feces. Factors like vitamin C intake, gut health, and calcium levels can influence how much oxalate is absorbed. Once absorbed, it is either used in metabolic processes or eliminated via the urine.
Relationship with Kidney Function
Kidneys filter blood and remove waste products, including oxalates, from the body. High oxalate levels can lead to calcium oxalate crystals in the kidneys, forming kidney stones. These stones can cause severe pain and may require medical treatment if they don’t pass naturally. Maintaining hydration by drinking ample fluids can mitigate the risks of stone formation.
Oxalate Toxicity
Excessive oxalate can lead to toxicity, though it is rare under normal dietary circumstances. Symptoms of oxalate toxicity may include joint pain, skin rashes, and difficulty breathing. Generally, the body efficiently regulates oxalate levels, preventing toxicity under typical dietary conditions.

Biological Implications and Research
While oxalates are simple molecules, their implications for biology and health are profound, prompting extensive research.
Oxalates in Pharmaceutical Studies
In medical science, understanding the precise role of oxalates can facilitate the development of new treatments for kidney stones and related conditions. With advances in research, therapeutic interventions are being refined to help manage oxalate levels, addressing both acute and chronic impacts.
Potential Role in Plant Science
In botany and agriculture, studying oxalates provides insights into plant physiology and their interactions with the environment. Selective breeding or genetic modifications might allow for crops with tailored oxalate levels, addressing both health concerns and agricultural needs.
Future Directions
Current research continues to unravel the complexities of oxalates and their multifaceted roles. As technology and science progress, novel approaches may further elucidate how these compounds can be managed effectively to improve human and environmental health.
Practical Tips for Managing Oxalate Intake
For those seeking to manage oxalate intake while maintaining a balanced diet, here are some simple strategies:
-
Diverse Diet: Avoid eating large amounts of high-oxalate foods in one meal. Instead, incorporate a wide range of nutrient-rich, low-oxalate options.
-
Calcium-Rich Foods: Pair high-oxalate foods with calcium-rich items like dairy to potentially reduce oxalate absorption.
-
Stay Hydrated: Drinking water is crucial to support the kidneys in filtering out oxalates and preventing stone formation.
-
Cooking Methods: Opt for boiling certain vegetables to reduce their oxalate content rather than eating them raw.
-
Monitor Vitamin C Intake: Large doses of vitamin C can increase oxalate production in the body. Moderation is key.

Conclusion
By exploring the chemistry of oxalates, you gain insight into a tangible aspect of biochemistry with concrete implications for diet and health. Though simple in construction, oxalates have varied and complex roles in both the plant and animal kingdoms. From how you prepare a meal to how you manage certain medical conditions, understanding oxalates can shape practical decisions and lead to better health outcomes. As research progresses, greater knowledge of these compounds promises to enrich our understanding of nutrition and the environment, influencing the choices you make tomorrow.